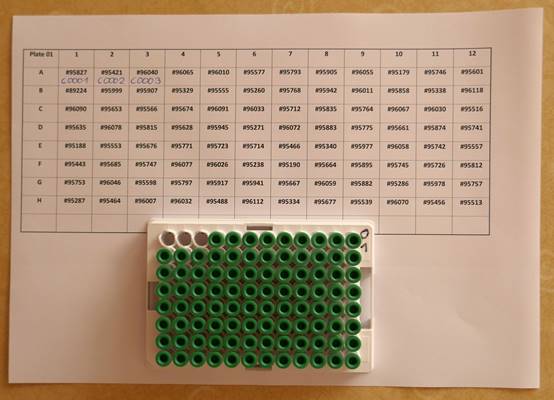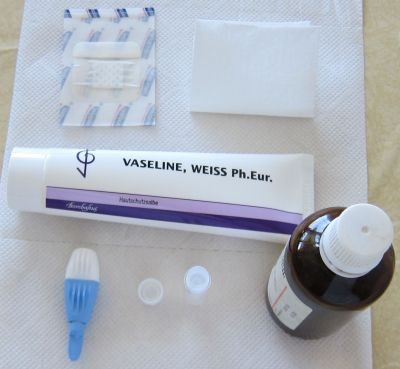

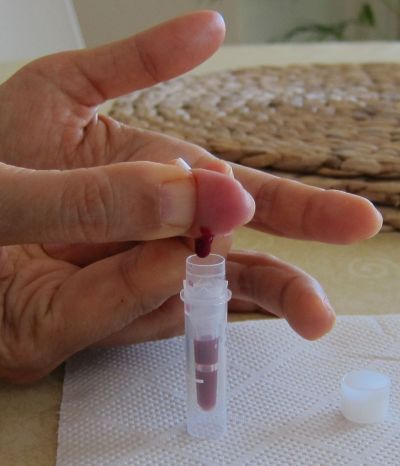

  |  |  |
The pictures above show an example on how capillary blood samples from the finger can be collected into a serum tube. The finger is first disinfected with alcohol, optionally a thin layer of vaseline or silicon spray added and then the finger punctured. After that the blood usually just drops into the serum tube (e.g. the one from Sarstedt which is shown here, ord. no. 20.1308) when the finger is a bit pressed as in this example (not milked). After some time in upright position (20-60 min) the tube can be centrifuged in a mini centrifuge (also available with power supply from 4 AA batteries) or a manual centrifuge (more expensive and not so convenient). To increase the blood volume warming the hand, using high efficient lancets (e.g. the blue ones from Becton Dickinson, ord. no. 366594 ), wiping off the blood with a tissue to initiate again the blood flow or adding vaseline/silicon spray to inhibit the coagulation of blood in the puncture hole can be helpful procedures. Getting 100 ul serum is usually no problem and can be easily pipetted with an inexpensive 100 ul pipette (around 25USD). Alternatively 50 ul serum and a 50 ul pipette would also be OK.
Storage of serum samples
To make the measurement easier and more reliable
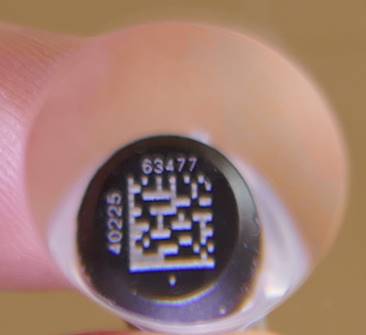
please provide samples only in 0.5 mL tubes from Micronic which are provided by
VitMin Lab. The important is that the tubes are correctly closed with the Push Caps.
For
sending the tubes to the lab itís not necessary to put them in boxes. A simple
Ziploc bag which needs the smallest volume can be often the best option.
The volume of the serum should be between 50 and 100 ul serum (the best is when the volume in all tubes is approximately the same). With volumes less than 25 ul a measurement might not be possible anymore and volumes above 100 ul causes various handling problems which can also have the risk that a measurement is not possible or errors are increased due to carry over effects.

 |
 |
 |
 |